Effect of Brazilian green propolis in patients with type 2 diabetes :A double-blind randomized placebo-controlled study
 INTRODUCTION
INTRODUCTION
Diabetes is a syndrome presenting with chronic hyperglycemia arising from insufficiency of insulin activity. Insulin resistance is defined as a decreased response of the peripheral tissues to insulin activity. Individuals with insulin resistance are predisposed to developing type 2 diabetes mellitus. Previous studies have revealed that the plasma concentration of inflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and high sensitivity C reactive protein (hsCRP) is increased in the insulin resistant states of obesity and type 2 diabetes.
Propolis is a resinous material collected by the Apis mellifera bee from leaf buds and cracks in the bark of various plants. Propolis contains a variety of chemical compounds, including polyphenols, flavonoids, amino acids, vitamins and caffeic acid phenethyl ester. Propolis presents numerous biological and pharmacological properties, such as immunomodulatory, antitumor, anti-inflammatory and anti-oxidant activity. In previous years, a number of studies have identified that propolis has hypoglycemic effects in animal models with type 2 diabetes. To the best of our knowledge, however, there have been no studies regarding these effects in human.
The aim of the present paper was to evaluate the effect of the Brazilian green propolis extract on glucose metabolism, renal function, lipid metabolism and inflammatory cytokines in patients with type 2 diabetes.
Materials and methods
Enrolled participants. Patients with type 2 diabetes were screened and enrolled if they were aged 35-80 years and received treatment with diet and exercise, oral hypoglycemic agents or glucagon like peptide-1 receptor agonists at the Kyoto Prefectural University of Medicine (Kyoto, Japan).
All the patients provided details of their demographics, medical history and medication usage. Body mass index was calculated asweight in kilograms divided by height in meters squared. Type 2 diabetes was diagnosed according to the Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Nephropathy was graded as follows: Normoalbuminuria, urinary albumin excretion (UAE) <30 mg/g of creatinine (Cr); microalbuminuria, 30-300 mg/g Cr; and macroalbumiuria, >300 mg/g Cr.
The patients treated with insulin were excluded. In addition, the following patients were also excluded: Severe renal dysfunction [estimated glomerular filtration rate (eGFR) <30 ml/min/1.73m2] and/or hepatic dysfunction (aspartate aminotransferase >100 U/l or alanine aminotransferase >100 U/l), as well as pregnant females. The study was examined and approved by the Institutional Review Board (IRB) and was subsequently implemented in accordance with Good Clinical Practice. The investigator or sub investigator informed each candidate patient of the study design using the leaflet and consent form authorized by the IRB prior to enrolling the patient in the study, and the patient consent was obtained in writing.
Study design and methods. The present study was a randomized, double blind study. For allocation of the participants, the numbered container method was used. The placebo served as a reference drug for comparison.
The propolis group received Brazilian green propolis (226.8 mg, 8.4 kcal/day), whereas the placebo group received tablets containing safflower oil, wheat germ oil and perilla oil (8.4 kcal/day). Brazilian green propolis was provided by the Yamada Bee Company, Inc. (Okayama, Japan). In each group, oral medication was administered once a day for 8 weeks. The diabetic diet and exercise regimen at baseline was continued, and was not changed during the study.
The primary outcome was the change in homeostasis model assessment of insulin resistance (HOMA-IR) at the end of the study. Secondary outcomes were the changes in fasting plasma glucose, glycated hemoglobin (HbA1c), serum insulin, total cholesterol, high-density lipoprotein, low density lipoprotein, triglyceride, remnant like particle lipoprotein cholesterol, uric acid, eGFR, TNF-α, IL-6, hsCRP, urine pH and UAE.
Biochemical analysis. Laboratory tests (hematology, biochemistry and urinalysis) were carried out before and 8 weeks after the start of taking tablets. Fasting blood and urine samples were obtained in the morning. The data of the laboratory tests were measured at a central laboratory institute at the Kyoto Prefectural University of Medicine. Fasting plasma glucose and insulin levels were used to calculate HOMA-IR, as previously reported. HbA1c was assayed using high performance liquid chromatography and was expressed as a National Glycohemoglobin Standardization Program unit. eGFR was calculated using the Japanese Society of Nephrology equation: eGFR = 194 x Cr-1.094 x age-0.287 (ml/min/1.73m2) for males, and for females the eGFR was multiplied by a correction factor of 0.739. Urinary albumin and Cr concentrations were determined using early morning spot urine. UAE was measured with an immunoturbidimetric assay.
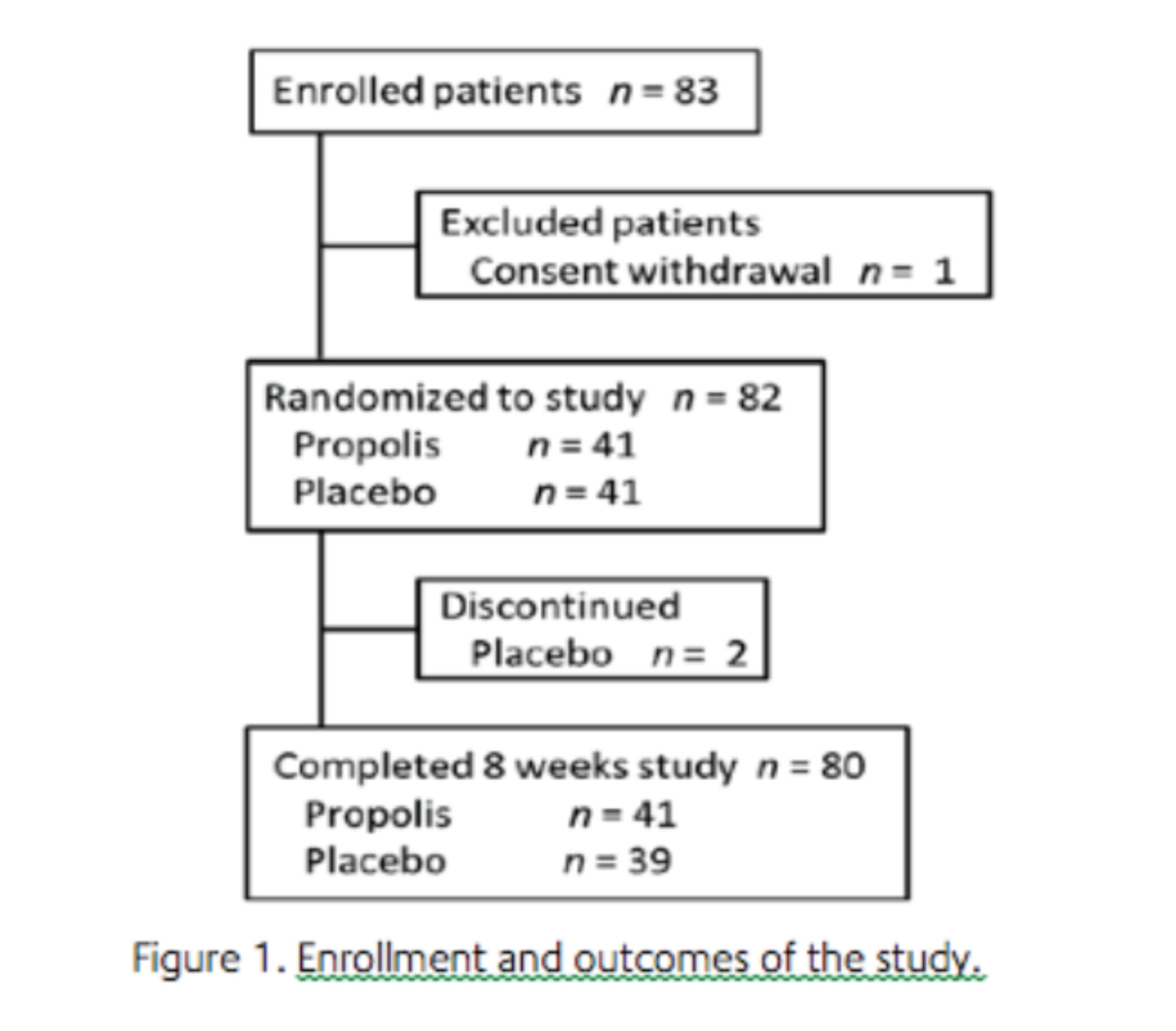
Statistical analysis. With a study sample of 82 patients (41 in each group), the study was estimated to have 80% power to detect a clinically important difference in the absolute difference in HOMA-IR of -1 between the two groups, assuming a standard deviation (SD) of 1.4 and a two-sided type one error rate of 5%.
The mean values are expressed as the mean ± SD. All the statistical tests were two tailed and P<0.05 was considered to indicate a statistically significant difference. As triglycerides, hsCRP and UAE showed skewed distributions, the data were normalized by logarithmic transformation for further statistical analysis. The differences in categorical variables between the two groups were evaluated using Fisher's exact test. Continuous variables were compared using a Student's t-test and a Mann-Whitney U test if appropriate for non-normally distributed data. All the statistical analyses were carried out using the JMP software, version 10.0 (SAS Institute Inc., Cary, NC, USA).

Efficacy. In the control group, HOMA-IR changed from 2.64±1.39 at baseline to 2.73±1.52 at 8 weeks after administration of the placebo. In the propolis group, HOMA-IR changed from 2.34±1.36 at baseline to 2.33±1.56 at 8 weeks after administration. The mean difference of HOMA-IR, which was a primary outcome between the baseline data and the data at 8 weeks after administration, was not significant between the two groups (P=0.62) (Table II). Furthermore, each group showed no significant change in HOMA-IR 8 weeks after the intake of propolis or placebo compared to the baseline.
All the parameters of glucose metabolism, renal function, lipid metabolism and inflammatory cytokines, which were secondary outcomes between baseline data and data at 8 weeks after administration, between the two groups did not have significant differences (Table II). However, it is notable that the concentration of uric acid in the blood of patients taking the placebo significantly increased 8 weeks later compared to the baseline (P<0.05; Table II), while in patients taking propolis it was maintained at a level similar to the baseline but did not significantly increase 8 weeks later (P=0.80; Table II).
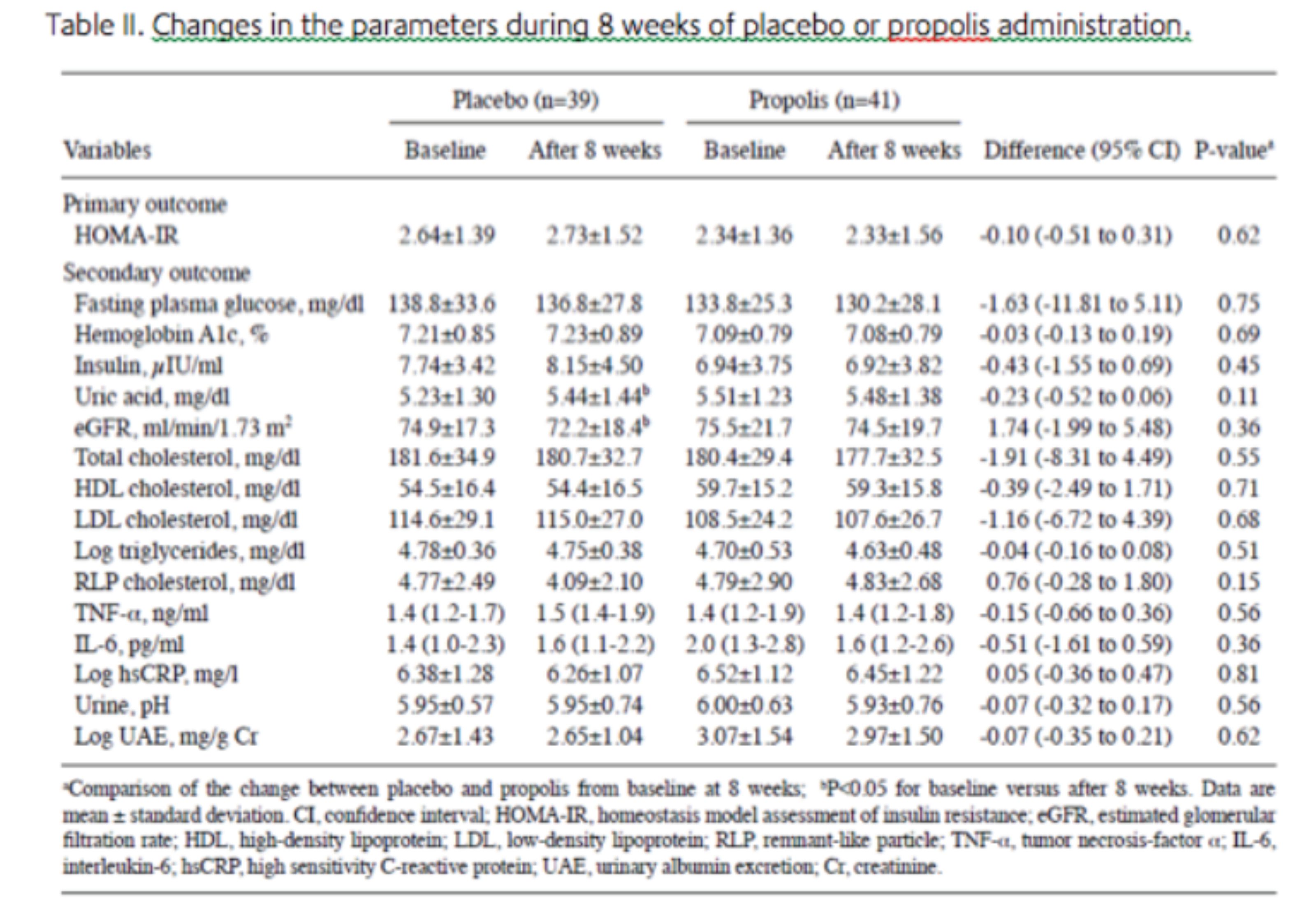
Furthermore, eGFR was decreased in patients taking placebo 8 weeks later compared to the baseline (P<0.01; Table II), while eGFR in patients taking propolis was maintained at a similar level to the baseline without any decrease 8 weeks later (P=0.52; Table II). Although TNF-α did not significantly increase in either group, patients taking the placebo showed a tendency to increase TNF-α (P=0.08; Table II), the value of which in patients taking propolis was stable (P=0.98; Table II). No reports on side effects, including allergy, were noted in patients who finished the protocol.
Discussion
The present study examined the efficacy of Brazilian green propolis compared to the placebo for 8 weeks in Japanese patients with type 2 diabetes. To the best of our knowledge, this is the first double blind randomized placebo-controlled study to investigate the effectiveness of propolis in patients with type 2 diabetes. In the study, there were no evident differences between the propolis and the placebo groups in the changes of HOMA-IR, HbA1c, fasting blood glucose or serum insulin level, so therefore, intake of 226.8 mg propolis/day for 8 weeks did not improve glucose metabolism in the patients with type 2 diabetes. However, as shown in Table II, the concentration of uric acid in the blood of patients taking propolis for 8 weeks was maintained at a level identical to the baseline without any significant increase (P=0.80; Table II), while for the patients taking the placebo, the concentration significantly elevated 8 weeks later (P<0.05; Table II). Furthermore, intake of 226.8 mg propolis/day did not improve eGFR, but maintained eGFR at the baseline without any decrease for 8 weeks (P=0.52; Table II). By contrast, eGFR of diabetic patients taking the placebo decreased 8 weeks later compared to the baseline (P<0.01; Table II). Furthermore, daily intake of propolis maintained TNF-α at a stable value for 8 weeks without any significant elevation (P=0.98; Table II), while diabetic patients taking the placebo showed a tendency to increase TNF-α (P=0.08; Table II). These observations suggest that daily intake of 226.8 mg propolis for 8 weeks prevents diabetic patients from developing a worse renal glomerular filtrating function and elevation of blood uric acid, and may have an anti inflammatory action.
The biological action of propolis originates from its active constituents, which differ in type and amount in the various types of propolis. Brazilian propolis represents 10-15% of the worldwide production and Brazil is the third world producer behind Russia and China (15). Among the types produced in Brazil, green propolis prevails and gains preference in the world propolis market. In the present study, the high quality Brazilian green propolis was used. Although toxic data for propolis are limited, various allergens have been isolated from propolis; such as 3-methyl-2-butenyl caffeate, phenylethyl caffeate, benzyl caffeate, geranyl caffeate, benzyl alcohol benzyl cinnammate, methyl cinnammate, ferulic acid and tectochrysin. Therefore, allergic reactions were of concern in the present study; however, no patient in the propolis group suffered from allergic reactions.
Multifold pathways, including increased polyol pathway flux, overactivity of the hexosamine pathway, increased formation of advanced glycation end products and activation of protein kinase C isoforms, are involved in diabetic complications. Increased reactive oxygen species inducing hyperglycemia-activated electron-transport chain in mitochondria are believed to be a main underlying mechanism linking all of these factors, and oxidative stress is possibly involved in the progression of pancreatic β-cell dysfunction. Propolis has a strong antioxidative activity and is confirmed to inhibit the increase of the malonaldehyde (MDA) level and improve antioxidase activity in the animal model and patients. Previous studies have reported that propolis can prevent oxidative stress-induced tissue damage by decreasing the overproduction of MDA and superoxide anion, and by restoring the respiratory control ration in mitochondrial tissue. Therefore, propolis may improve glucose metabolism by an attenuation of mitochondrial oxidative stress.
In addition, extracellular pH plays an important role in glucose metabolism. In a rat skeletal muscle-derived cell line, L6 cell, the phosphorylation level of the insulin receptor and Akt is significantly diminished in low pH media, and binding of insulin to its receptor on the plasma membrane is reduced by lowering the extracellular pH, while the expression of insulin receptors on the plasma membrane is not affected. As a result, insulin stimulated 2-deoxyglucose uptake in L6 cells is diminished in low pH media. An ex vivo study by Yamauchi et al reported that insulin mediated 2-deoxy-D-glucose uptake by rat soleus muscle is inhibited by a reduction in the pH of the medium from 7.4 to 6.8. Furthermore, Aoi et al have reported that a propolis contained diet improved, by increasing, the pH of ascites and metabolic tissues compared to the normal diet and improved glucose metabolism in Otsuka Long-Evans Tokushima Fatty rats, whose pH in various tissues was lower compared to normal rats. Therefore, propolis may improve insulin sensitivity by an increase of extracellular pH.
In the present study, at least two limitations should be noted. First, the trial may have been too short to observe the change of glucose metabolism. Propolis may require a longer duration to improve glucose metabolism in humans, so administration of propolis for 8 weeks appears to be unlikely to elicit significant improvements in HbA1c. Second, the dose of propolis may not have been sufficient. Numerous studies report the efficacy of propolis on diabetes; however, the majority are animal experiments and administered doses of propolis were extremely high (such as 50-300 mg/kg of the body weight) (8-10,28-30). Therefore, if a higher dose of propolis was administered to patients, the effect of propolis on glucose metabolism may take effect.
In conclusion, 226.8 mg/day of Brazilian green propolis for 8 weeks prevented the actions of hyperuricemia and dysfunction of renal glomerular filtrating function that commonly develop in patients suffering from diabetes mellitus. Therefore, further clinical studies should be continued to verify whether much higher doses and/or longer administration of Brazilian green propolis are useful in the prevention and care of diabetes mellitus.

