Nutrition And Cancer
 INTRODUCTION
INTRODUCTION
Prostate cancer is the most common non-skin cancer and has the second highest cancer death rate in men (1). Age, family history of prostate cancer, and race are the only well-established risk factors for the disease. Identifying modifiable risk factors could help decrease the incidence and mortality rates of prostate cancer. Saw palmetto’s use in the treatment of benign prostatic hyperplasia (BPH) (2,3) and its purported mechanism of action have prompted researchers to consider its possible efficacy in decreasing the risk of prostate cancer.
Saw palmetto supplements are derived from the berries of the plant Serenoa repens, which is native to the South Atlantic coast of the United States and other areas. About 2% of American men use saw palmetto supplements (4). Popular saw palmetto supplements are available as encapsulated, powdered berries in doses of 900 mg or above or as a lipid and sterol extract in doses of about 160 mg (5). Although BPH is not considered a precursor for prostate cancer pathologically, 83% of prostate cancers occur in men with BPH (6), and both conditions respond to anti-androgen treatment and share common risk factors (7). Saw palmetto is thought to treat BPH by inhibiting the 5-α-reductase enzyme (8–17), which irreversibly converts testosterone to the more potent androgen dihydrotestosterone (DHT), although conflicting evidence exists (18–20). The Prostate Cancer Prevention Trial found that treatment with finasteride, a strong 5-α-reductase inhibitor also used to treat BPH, decreased the risk of developing prostate cancer (21). In vitro studies have described other mechanisms of action for saw palmetto, such as inhibiting DHT from binding to androgen receptors (22,23), diminishing estrogen (24), and decreasing inflammation (25–27). These associations have prompted scientists to investigate whether saw palmetto is active against prostate tumors in in vitro studies. Saw palmetto lipidosterolic extract appears to inhibit the growth of LNCaP (27–31), PC-3 (29,31), 267B-1 (27), and BRFF-41T (27) cancerous human prostatic cell lines in vitro when used in physiologically plausible doses (32). This extract has been found to inhibit cancerous prostate cell growth through several different mechanisms, including apoptosis (27,30), necrosis (30), and growth inhibition (27–29). Saw palmetto also appears to target prostate tissue. Active compounds in saw palmetto extract, when taken orally, accumulate in the prostate gland preferentially over other organs in rats (33), although no studies have examined saw palmettos activity against prostate cancer in rodent models.
The only human studies involving saw palmetto and prostate cancer have been on the use of saw palmetto for treatment of prostate cancer, including clinical studies of the saw palmetto–containing formula PC-SPES (34–36), and two case reports of aggressive prostate cancer treated with a combination of saw palmetto and other supplements in the absence of conventional treatment (37,38). PC-SPES includes eight herbs and was withdrawn from the market in 2002 due to contamination with synthetic estrogens (39), thus limiting our ability to speculate on the role saw palmetto may play relative to the other compounds in the formula. The subjects treated with saw palmetto in the case reports improved markedly, but the use of additional compounds, including lycopene, and the positive bias in the publication of case reports limit the conclusions we can draw from them about saw palmetto and prostate cancer. Given the fairly common use of saw palmetto and the evidence that it has activity in the prostate, it is important to understand whether saw palmetto supplement use decreases, increases, or has no effect on the risk of developing prostate cancer. We examined this association in a large cohort of older U.S. men that specifically recruited supplement users. To our knowledge, this is the first epidemiological study of saw palmetto use and prostate cancer risk.
Methods
Study Population and Design Subjects included men who enrolled in the Vitamins and Lifestyle (VITAL) study, a longitudinal cohort study of 50- to 76-yr-old individuals living in the 13 western counties of Washington state. Details of recruitment, data collection, and follow-up procedures can be found in Ref. 40. Briefly, recruitment began in October 2000 and was completed in December 2002. Questionnaires (195,465) with a cover letter targeting supplement users were sent to men whose names were obtained from a commercial mailing list; 37,382 men (19.1%) returned questionnaires that passed eligibility and quality-control checks. Subjects with a self-reported history of prostate cancer at baseline (n = 2,013), who developed noninvasive prostate cancer (n = 2), or with incomplete answers regarding history of prostate cancer (n = 128) or saw palmetto use (n = 68) were excluded, leaving 35,171 men for analysis.
EXPOSURE ASSESSMENT
Subjects provided detailed information about demographic characteristics, supplement use, medications, medical history, and diet in a 24-page mark-sense questionnaire. Assessment of saw palmetto from supplements and multivitamins: Respondents were asked about regular intake (defined as at least once a week for a year) of saw palmetto both as part of amultivitamin (defined as containing 10 ormore vitamins and/orminerals) and as a single supplement or a part of othermixtures (referred to from here on as “single supplements” for simplicity). Respondents were asked to report the brand name of their multivitamin and whether it contained saw palmetto.We only asked about herbals in multivitamins taken currently because multivitamins contain too many compounds for subjects to reliably identify previous herbal constituents. For the single-supplement and multivitamin forms of saw palmetto, respondents were asked to report the number of times perweek (1–3, 4–6, or 7) and the number of years in the past 10 (1–2, 3–5, or 6+) that they had taken it and if theywere currently taking it.We did not ask about dosage per day because of the variation in constituent ratios and the disparity between the stated and actual dose of many saw palmetto supplements (41,42).We computed the average use of saw palmetto over the 10 yr prior to baseline as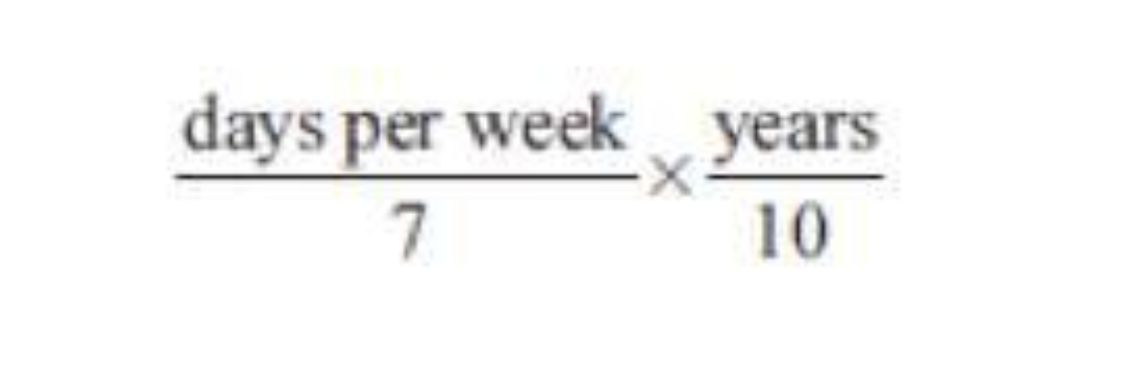
summed over saw palmetto single supplements and saw palmetto in multivitamins.We weighted saw palmetto–containing multivitamins as one-tenth of the value of saw palmetto single supplements to account for the average difference in dose between multivitamins and saw palmetto single supplements that we found on the market. Respondents were asked to look at their supplement bottles when completing the form. The multivitamin portion of the VITAL study questionnaire was found to be valid and reliable (43).
ASSESSMENT OF COVARIATES:
Information about possible confounders and effect modifiers was collected via the questionnaire. These factors included whether no, one, or two or more first-degree relatives [father and/or brother(s)] had had prostate cancer; use of testosterone or medications for BPH (finasteride, doxazosin, and tamsulosin) in the previous 2 wk; and medical tests and conditions, including a prostate specific antigen (PSA) test in the previous 2 yr, prostate biopsy, or transurethral resection of the prostate, and physician - diagnosed BPH. Additional potential confounders, including age, ethnicity, marital status, income, education, anthropometrics, physical activity, and usual diet and supplement intake, were also recorded in the questionnaire.
FOLLOW-UP FOR CANCER AND CENSORING
We identifiedmen diagnosed with prostate cancer through December 2003 through linkage to the western Washington Surveillance, Epidemiology, and End Results (SEER) cancer registry, which records new diagnoses of cancer, including their stage and grade, in the 13 counties of the Puget Sound. Information is obtained from all hospitals in the area and from pathologists, oncologists, radiotherapists, and state death certificates. During the approximately 2-yr follow-up period (75,755 person-years), 580 men developed invasive prostate cancer. Cancer grade was classified as Gleason score of 2–6 (low/moderate grade) vs. Gleason score of 7–10 (high grade). This classification scheme was used by SEER beginning in January 2003, and data on Gleason scores from cancers in earlier years were reabstracted and recoded into these categories.
We obtained information on deaths by linkage to Washington state death records and, for moves out of the SEER registry catchment area, by linkage to the U.S. National Change of Address System. Men were censored at the earliest of date of prostate cancer diagnosis (1.7%), date withdrawn from the study (0.02%), date moved out of the SEER coverage area (3.0%), date died (2.0%), or date of last cohort follow-up (December 31, 2003; 93.3%).
STATISTICAL ANALYSES
We used Cox proportional hazard models to calculate the hazard ratios and 95% confidence intervals (CIs) of prostate cancer by categories of saw palmetto use. Age was used as the time variable, effectively controlling for age in all analyses. We evaluated as potential confounders PSA testing, family history of prostate cancer, BPH, prostate biopsy, dietary and supplemental lycopene and selenium intake, testosterone andmedications for BPH, education level, race/ethnicity, and servings of fruit and vegetables.We controlled for history of BPH (yes or no) and selenium supplement intake (quartiles) because only inclusion of these factors changed the hazard ratio coefficient by more than 10%. We chose to control for family history of prostate cancer (none, one first-degree relative, and two or more first-degree relatives) a priori because of its well-established relationship with the risk of prostate cancer. To explore whether the association between saw palmetto and prostate cancer differed by another variable (for example, BPH, age, or PSA testing), we conducted analyses stratified on each variable and also evaluated the coefficients for the cross-products term (saw palmetto × effect modifier) using the likelihood ratio test. The association between PSA testing and prostate cancer differed by age; therefore, we controlled for PSA testing as a stratification variable in the Cox model. To test for a trend across categories, we modeled a single linear variable. Additional models looked at the associations of saw palmetto by disease subgroups (Gleason score of 2–6 vs. Gleason score of 7–10). We examined Schoenfeld residuals to evaluate the proportional hazards assumption. In the models presented, there was no evidence that this assumption was violated. The two-tailed P value of 0.05 was considered statistically significant. All statistical analyses were conducted using STATA version 8.2 (College Station, TX).
Results
Table 1 gives distributions of the cohort overall and of the men who used saw palmetto by demographic characteristics, family history, and medical history. Overall, 10.3% of the men in the cohort (n = 3,625) used saw palmetto at least once a week for a year in the 10 yr prior to baseline. Saw palmetto users were more likely to be older and to have had more education, a PSA test in the 2 yr before baseline, BPH, and a prostate biopsy. Furthermore, men taking saw palmetto were more likely to have taken drugs for BPH in the 2 wk before baseline as well as other dietary supplements, especially selenium, vitamin E, and lycopene (data not shown).Men with a family history of prostate cancer were only slightly more likely to take saw palmetto supplements.
Table 2 gives the hazard ratios for the four saw palmetto intake variables: ever used, years of use in the past 10, frequency of use (days/week) in the years used, and average 10-yr use. Controlling for only age, family history of prostate cancer, and PSA testing, saw palmetto intake of at least once a week for a year was associated with a 16% increased risk (95% CI = 0.91–1.47). After additionally controlling for BPH (HR = 1.03; 95% CI = 0.80–1.30) and quartiles of selenium use (HR = 0.95; 95% CI = 0.74–1.23), the hazard ratios were attenuated. There was no indication of a lower relative risk with increasing years of use, days per week of use, average 10-yr use categorized as above and below the median (Table 2), or average 10-yr use categorized into quartiles (data not shown). There was no clear difference in the association of saw palmetto with prostate cancer by grade of cancer (Table 3). Two other subgroups, PSA testing within the previous 2 yr and BPH at baseline, were analyzed similarly. Neither showed significant effect modification (data not shown).