International Journal Of Molecular Sciences
Head and neck cancers include cancers evolved from the oral cavity, pharynx, larynx, paranasal sinuses and nasal cavity, and salivary glands. Head and neck cancers rank as the sixth most common cancer worldwide, affecting 650,000 people and causing 350,000 deaths per year [1,2]. Oral cancer is the most common type of head and neck cancer and caused 135,000 deaths worldwide in 2013 [3]. According to the Surveillance, Epidemiology, and End Results Program (SEER), the five-year survival rate of oral cavity and pharynx cancer in the United States is 63%. The poor prognosis of oral and oropharyngeal squamous cell carcinoma (OSCC), which account for 90% of the oral cancers, is due to the low response rate to current therapeutic drugs [2,4]. Propolis (bee glue) is produced by honeybees through mixing the secretions of their hypopharyngeal glands with the digested product of resins collected from leaves, flowers, and tree barks, which is used to build honeybee hives [5]. Propolis protects honey bee hives against rain and is a very sticky substance that prevents insects, rodents, and robber bees from entering the hives [5]. Propolis also acts as a biocide to kill invasive bacteria, fungi, or even larvae [5]. Propolis is a natural medicine used for hundreds of years and is being sold as dietary supplements. Propolis has been reported to exhibit anti-bacterial, anti-viral, fungicidal, anti-oxidative, free radical scavenging, immuno-modulatory, and anti-cancer activities [5]. Clinical trials, animal models, and cell culture experiments indicated that treatment with propolis is beneficial in dental application [5,6], such as decreasing the dentinal hypersensitivity [7,8], defending the dental caries [6], decreasing the oral mucositis resulted from chemotherapy [9–11], fortifying the salivary gland function [12], reducing the xerostomia due to radiotherapy to salivary glands [12], preventing oral cancer [13], inhibiting plaque and having anti-inflammatory effects [14], increasing the periodontal ligament cell viability of avulsed teeth [15], stimulating wound healing in the dental pulp [16], acting as an analgesic [17], and as an antibacterial agent against oral pathogens [18–20], declining the quantity of Enterococcus faecalis in root canals [21], lessening gingivitis [22], reducing recurrent aphthous stomatitis (RAS) [23,24], protecting the oral mucosa [25], and promoting wound healing after surgeries in the oral cavity [14]. Propolis is a complex mixture of more than 300 different natural constituents including phenolic acid, terpenes, cinnamic acid, caffeic acid, aromatic aldehydes, alcohols, amino acids, fatty acids, vitamins (A, B1, B2, B3, and B7), esters, minerals, essential oils, and flavonoids (flavones, flavonols, and flavanones) [5]. The component of propolis varies according to the species of bees, the difference of geological region, the different kinds of plants and flowers the bees collect, which makes it difficult to define the actual molecular mechanisms of the anti-cancer activity of propolis. Caffeic acid phenethyl ester (CAPE) (Figure 1) is one of the most bioactive components extracted from honeybee hive propolis [26,27]. CAPE treatment exhibits anti-carcinogenic, anti-inflammatory, anti-viral, and immuno-modulatory properties [28]. Several recent studies indicate that CAPE treatment suppresses proliferation, survival, and invasion of human oral cancer cells. We therefore discuss the potential of using CAPE as a treatment for patients with oral cancer in this review article.
Materials and Methods
There are several types of oral cancers. The majority (>90%) of oral cancers are OSCC [2,4,29]. OSCCs arise in the oral cavity, oropharynx, larynx or hypopharynx and are characterized by poor prognosis and low survival rate [30]. The incidence and mortality rates worldwide of OSCC are approximately 5.9 and 3.3 per 100,000 persons per year, respectively [31]. Forty thousand OSCC cases were diagnosed and 8000 patients died from OSCC in the United States in 2012 [29,32]. The incidence of oral cancer is highest in Eastern and Southern Asia as well as central African countries and oral cancer accounts for 40%–50% of all malignancies in South and South-East Asian countries [33,34]. Environmental carcinogens, such as betel quid chewing, tobacco smoking, and alcohol drinking, have been identified as major risk factors for head and neck cancers [35]. According to the of the fifth leading cause of cancer death in Taiwanese males in 2012. The majority of oral cancer patients in Taiwan are regular users of betel quid [35]. Betal quid is a combination of betel leaf, areca nut, and slaked lime [35]. The cumulative effect of betel quid chewing, alcohol drinking, and tobacco smoking increases the risk of oral cancer up to 123-fold in Taiwanese patients [35].
Caffeic Acid Phenethyl Ester (CAPE) and Anticancer
Effects
Caffeic acid phenethyl ester (CAPE) (Figure 1) is one of the main bioactive components extracted from honeybee hive propolis. CAPE is a strong antioxidant [26,27] and a lipophilic derivative of caffeic acid and a phenolic antioxidant structurally related to 3,4-dihydroxycinnamic acid. CAPE is a well-known NF-κB inhibitor [27]. CAPE treatment (50–80 µM) inhibits the activation of NF-κB via preventing the translocation of the p65 unit of NF-κB [27] and blocking the binding between NF-κB and DNA [27]. CAPE is an excellent anti-cancer agent. Treatment with CAPE inhibits the transformation of normal cells to cancer cells [36] as well as suppressing the proliferation of several human cancer cell lines, such as breast [37,38], prostate [39–42], lung [43,44], head and neck [45], cholangio [46], and cervical [47] cancer cells. Non-cancer human cells are much more resistant to CAPE treatment, indicating the potential selective cytotoxic effect against cancer cells of CAPE treatment [39,43,45,48]. CAPE treatment induces apoptosis or cell cycle arrest (G1 or G2/M) in different types of cancer cells [36,38,39,43,47,49–57]. CAPE treatment suppresses cancer cell movement and migration [58,59]. Oral administration or intraperitoneal (i.p.) injection of CAPE prevents cancer initiation, tumor growth, and cancer metastasis of colon, liver, and breast cancers [46,60–68] in animal models. CAPE is distributed extensively into animal tissues and is eliminated rapidly with a short half-life [69]. Intraperitoneal injection of CAPE at 10–30 mg/kg for seven days does not show toxic effects or affect the body weight of mice [65]. Additionally, CAPE treatment inhibits the proliferation of breast cancer stem cells [70]. CAPE treatments have also been shown to sensitize cancer cells to chemotherapeutic drugs and radiation treatment by inhibiting pathways that lead to treatment resistance as well as protecting important organs under chemotherapy and radiation treatments in animal models [71–81]. As CAPE exhibits very little or no toxic side effects, it is a potentially good candidate as a cancer therapeutic agent. Treatment with CAPE not only may suppress tumor growth in patients but also may protect patients from chemotherapy or radiation therapy.
Chemoprevention Effects of CAPE on Oral Cancer Cells
The epidermal growth factor receptor (EGFR), a receptor tyrosine kinase (RTK), is the cell-surface receptor for members of the epidermal growth factor (EGF) family. Elevated gene expression of EGFR has been reported to be associated with poor prognosis in OSCC [82,83]. Treatment with 5–30 µM CAPE dose-dependently suppresses the total abundance and phosphorylation of EGFR in breast cancer cells [37]. Cyclooxygenase (COX), including COX-1 and COX-2, is the enzyme responsible for the formation of the prostanoids. Cyclooxygenase-1 (COX-1) is present in most tissues, while Cyclooxygenase-2 (COX-2) is present at sites of inflammation. Both mRNA and protein level of COX-2 (also known as prostaglandin-endoperoxide synthase 2) are highly up-regulated in OSCC [84] and in high-risk premalignant oral lesions [85]. Up-regulation of COX-2 correlates to higher lymph node metastasis, faster cell proliferative activity, and worse survival rate in patients with oral carcinoma [86]. CAPE suppresses COX-2 activity with an IC50 of 6.3 nM in J774 macrophages [87]. Treatment with 35–70 µM of CAPE inhibits the activity and expression of COX-2 in human 1483 oral squamous carcinoma cells [88]. According to the facts that CAPE treatment is able to inhibit the activity and abundance of EGFR and COX-2, we believe that administration of CAPE can prevent and delay the development or progression of oral cancers.
Anticancer Activity Effects of CAPE on Oral Cancer Cells
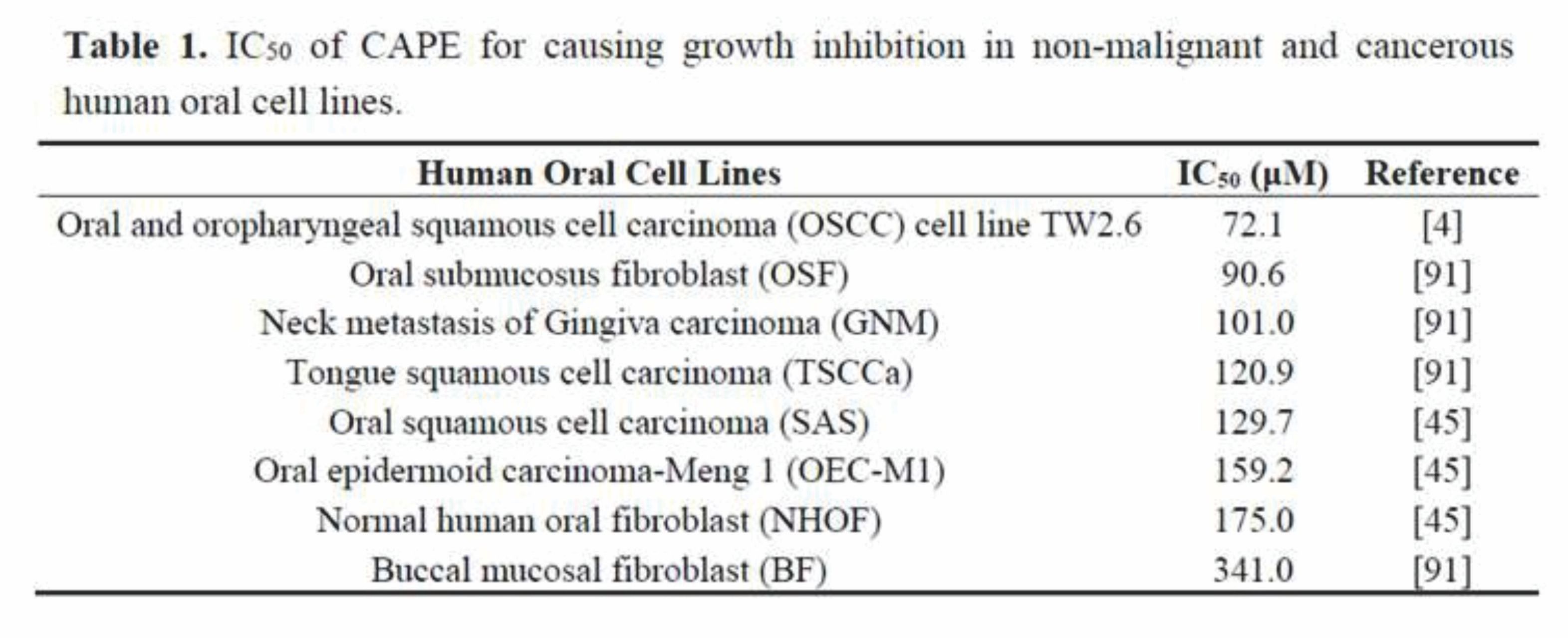 TW2.6 is an OSCC cancer cell line established from the untreated primary squamous cell carcinoma of the buccal mucosa from a 48-year-old Taiwanese male patient with a betel quid chewing and tobacco smoking habit [4,89]. TW2.6 cells have morphological features of keratinocytes with a doubling time of 24 h [4,89]. CAPE treatment dose-dependently suppressed the cell proliferation and soft-agar colony formation of TW2.6 cells [4], confirming the anticancer activity of CAPE against oral cancer cells. CAPE treatment suppressed the survival and growth of several head and neck cancer cell lines. CAPE treatment suppressed the proliferation of oral submucosus fibroblast (OSF), neck metastasis of Gingiva carcinoma (GNM), tongue squamous cell carcinoma (TSCCa), oral squamous cell carcinoma (SAS) cell line, oral epidermoid carcinoma-Meng 1 (OEC-M1) cell line, and OSCC cell line TW2.6 [4,45,90] when being treated for as short as 24 h (Table 1). The SAS cell line is derived from a human squamous cell carcinoma developed from the primary lesion of a tongue carcinoma in a Japanese patient. SAS cell line has wild-type p53, high invasive potential, and high migration ability [45,91]. The OEC-M1 cell line is a human oral epidermoid carcinoma generated from the lesion of an oral carcinoma in a Taiwanese patient, which is a p53 mutant, resistant to retinoic acid treatment, and expresses smaller sized hypophosphorylated Rb proteins compared with normal cells [45,92]. Normal human oral fibroblast (NHOF) cells and buccal mucosal fibroblast (BF) cells were more resistant to CAPE treatment with an IC50 of 175 and 341 µM, respectively [45,90], (Table 1), indicating that CAPE exhibits selective suppressive effect on human oral cancer cells. The suppressive effect of CAPE accumulated over time. The IC50 of CAPE to suppress proliferation of TW2.6 cells was 72.1, 41.5, and 19.0 µM for 24, 48, and 96 h of treatment, respectively. Although the IC50 of CAPE seems to be relatively high as compared to other chemotherapy drugs, CAPE can be applied in the form of topical cream or mouth rinsing solution. We believe that daily oral administration of CAPE for a week or longer is possible to cause regression of oral cancer cells.
TW2.6 is an OSCC cancer cell line established from the untreated primary squamous cell carcinoma of the buccal mucosa from a 48-year-old Taiwanese male patient with a betel quid chewing and tobacco smoking habit [4,89]. TW2.6 cells have morphological features of keratinocytes with a doubling time of 24 h [4,89]. CAPE treatment dose-dependently suppressed the cell proliferation and soft-agar colony formation of TW2.6 cells [4], confirming the anticancer activity of CAPE against oral cancer cells. CAPE treatment suppressed the survival and growth of several head and neck cancer cell lines. CAPE treatment suppressed the proliferation of oral submucosus fibroblast (OSF), neck metastasis of Gingiva carcinoma (GNM), tongue squamous cell carcinoma (TSCCa), oral squamous cell carcinoma (SAS) cell line, oral epidermoid carcinoma-Meng 1 (OEC-M1) cell line, and OSCC cell line TW2.6 [4,45,90] when being treated for as short as 24 h (Table 1). The SAS cell line is derived from a human squamous cell carcinoma developed from the primary lesion of a tongue carcinoma in a Japanese patient. SAS cell line has wild-type p53, high invasive potential, and high migration ability [45,91]. The OEC-M1 cell line is a human oral epidermoid carcinoma generated from the lesion of an oral carcinoma in a Taiwanese patient, which is a p53 mutant, resistant to retinoic acid treatment, and expresses smaller sized hypophosphorylated Rb proteins compared with normal cells [45,92]. Normal human oral fibroblast (NHOF) cells and buccal mucosal fibroblast (BF) cells were more resistant to CAPE treatment with an IC50 of 175 and 341 µM, respectively [45,90], (Table 1), indicating that CAPE exhibits selective suppressive effect on human oral cancer cells. The suppressive effect of CAPE accumulated over time. The IC50 of CAPE to suppress proliferation of TW2.6 cells was 72.1, 41.5, and 19.0 µM for 24, 48, and 96 h of treatment, respectively. Although the IC50 of CAPE seems to be relatively high as compared to other chemotherapy drugs, CAPE can be applied in the form of topical cream or mouth rinsing solution. We believe that daily oral administration of CAPE for a week or longer is possible to cause regression of oral cancer cells.
Conclusions
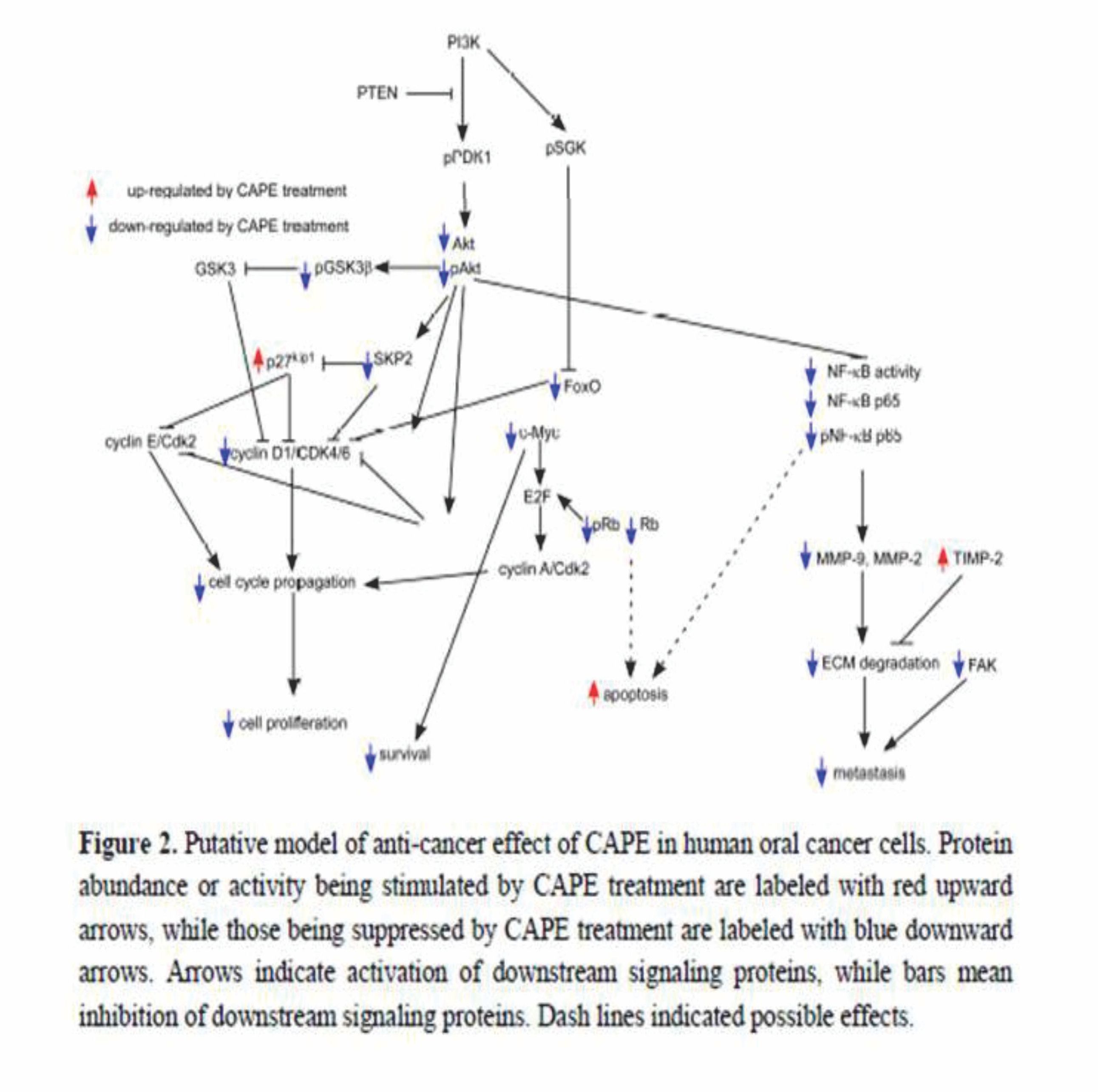
According to the above summaries in this review, there is strong evidence that CAPE treatment suppress proliferation, survival, metastasis, EGFR and COX-2 activity, PI3K-Akt signaling, and Skp2 in human oral cancer cells. We summarize the effects of CAPE treatment on different signaling proteins and the potential effect on cell survival, cell cycle, cell proliferation, and metastasis of oral cancer cells in Figure 2. The good bioavailability through the oral route, and the good historical safety profile of propolis, makes CAPE an ideal adjuvant agent for future oral cancer treatment. We believe that CAPE, being applied in the form of a topical cream or mouth rinsing solution, either alone or with 5-FU, can be a potentially effective treatment for patients with advanced oral cancer, targeting PI3K/Akt signaling, NF-κB, MMPs, and cell cycle regulatory proteins.